Efficacy is highly variant dependent. In Vitro studies suggest a lack of efficacy for omicron Haars, Liu, Pochtovyi, Sheward, VanBlargan. mAb use may create new variants that spread globally Focosi, Leducq, and may be associated with prolonged viral loads, clinical deterioration, and immune escape Choudhary, Günther, Leducq.
Recent:Wilcock.
Bamlanivimab/etesevimab was adopted
in 4 countries.
Submit updates/corrections .
May 19 |
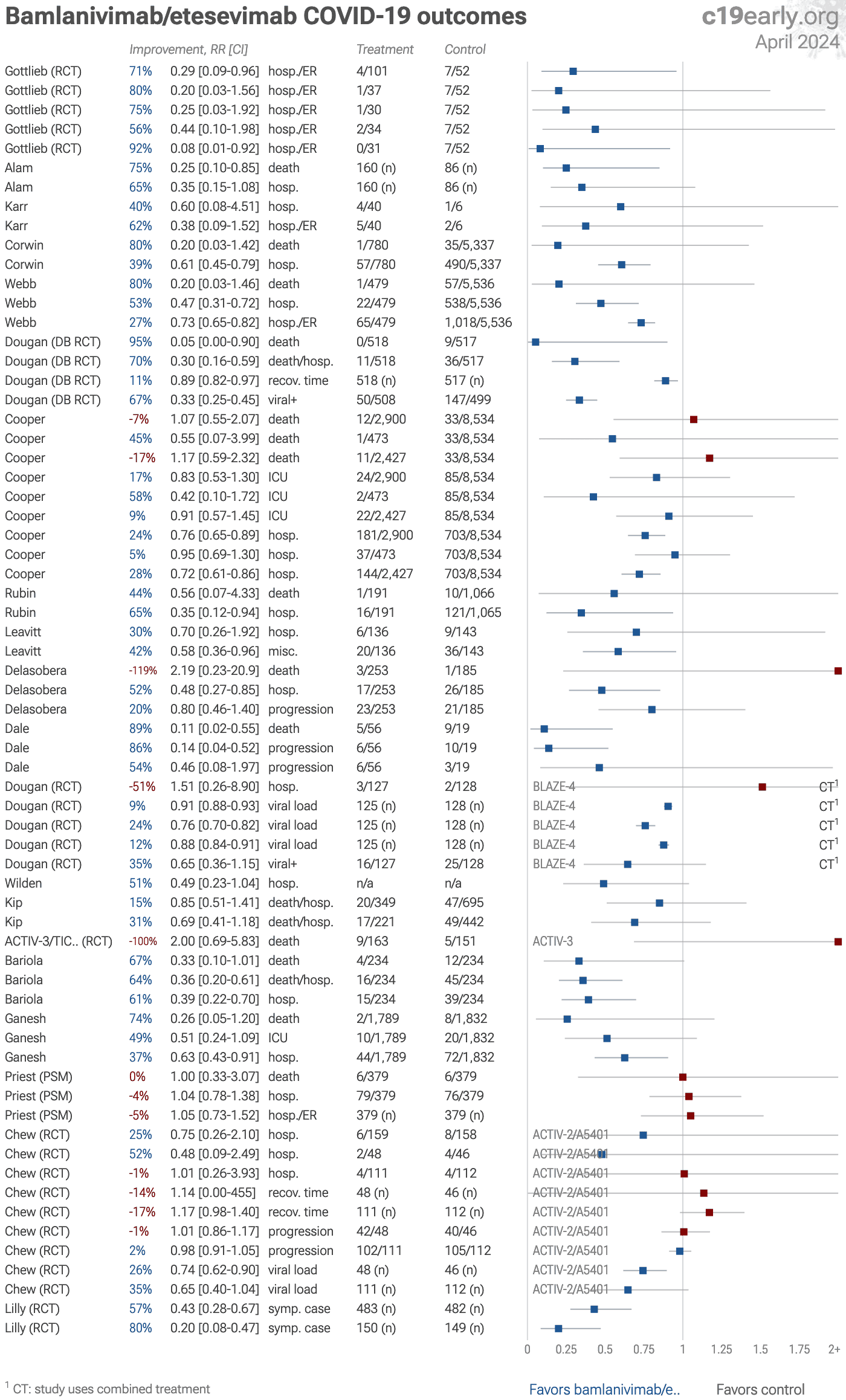
Bamlanivimab/etesevimab for COVID-19: real-time meta analysis of 20 studies | |
| Statistically significant lower risk is seen for mortality, hospitalization, recovery, cases, and viral clearance. 15 studies from 13 independent teams (all from the same country) show significant improvements. Meta analysis using the mos.. | ||
Jan 26 |
et al., JAMA Health Forum, doi:10.1001/jamahealthforum.2023.5044 | Clinical Risk and Outpatient Therapy Utilization for COVID-19 in the Medicare Population |
| Analysis of Medicare beneficiaries in 2022 showing that outpatient COVID-19 treatments like antivirals and monoclonal antibodies were disproportionately used by patients at lower risk of severe infection and outcomes. Retrospective studie.. | ||
Jan 7 |
et al., medRxiv, doi:10.1101/2024.01.06.24300890 | Variant-specific humoral immune response to SARS-CoV-2 escape mutants arising in clinically severe, prolonged infection |
| Report on a prolonged SARS-CoV-2 infection in an immunocompromised patient treated with bamlanivimab, where bamlanivimab escape variant S:S494P emerged. Later, the onset of an endogenous IgM antibody response coincided with the appearance.. | ||
Sep 28 2023 |
et al., Vaccines, doi:10.3390/vaccines11101533 | In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 |
| In Vitro study showing sharply reduced neutralization of SARS-CoV-2 variants XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 with monoclonal antibodies cilgavimab, tixagevimab, imdevimab, etsevimab, casirivim.. | ||
Sep 27 2023 |
et al., Microorganisms, doi:10.3390/microorganisms11102417 | Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023 |
| Analysis of 7,950 SARS-CoV-2 samples from central Sweden collected between March 2022 and May 2023 tracking the prevalence of omicron sublineages and mutations in the spike protein conferring resistance to monoclonal antibodies over time... | ||
Aug 10 2023 |
et al., Drug Resistance Updates, doi:10.1016/j.drup.2023.100991 | Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment |
| Review of reports of treatment-emergent resistance to COVID-19 monoclonal antibodies (mAbs), showing that some post-mAb treatment mutations appeared to spread globally soon after the mAb was introduced, raising concerns about transmission.. | ||
Jun 10 2023 |
et al., Value in Health, doi:10.1016/j.jval.2023.03.2056 | Variation in Demographic Characteristics, Socioeconomic Status, Clinical Presentation and Selected Treatments in Mortality Among Patients with a Diagnosis of COVID-19 in the United States |
| Retrospective analysis of mortality for COVID-19 patients in the USA. Authors do not provide adjusted results, preventing any strong evidence. However it is notable that, despite comparable treatment frequencies, the mortality for patient.. | ||
Apr 4 2023 |
et al., Annals of Internal Medicine, doi:10.7326/M22-1286 | Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 |
| 15% lower combined mortality/hospitalization (p=0.54). Retrospective 2,571 patients treated with mAbs in the USA, and 5,135 control patients, showing lower combined mortality/hospitalization for bamlanivimab, bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab, and bebtelovimab, with s.. | ||
Oct 15 2022 |
et al., American Journal of Health-System Pharmacy, doi:10.1093/ajhp/zxac295 | The impact of COVID-19 monoclonal antibodies on clinical outcomes: A retrospective cohort study |
| 61% higher mortality (p=0.4), 51% higher ICU admission (p=0.5), 49% lower hospitalization (p<0.0001), and 39% lower progression (p<0.0001). PSM retrospective 1,344 patients in the USA, showing lower hospitalization with monoclonal antibody treatment. Authors combine patients treated with bamlanivimab, bamlanivimab/etesevimab, or casirivimab/imdevimab. | ||
Aug 22 2022 |
et al., Nature Communications, doi:10.1038/s41467-022-32551-2 | Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19 |
| 25% lower hospitalization (p=0.6), 14% slower recovery (p=0.97), 1% higher progression (p=1), and 26% improved viral clearance (p=0.002). RCT 317 outpatients in the USA showing faster viral load and inflammatory biomarker decline, but no significant differences in clinical outcomes. | ||
Mar 31 2022 |
et al., Journal of the National Comprehensive Cancer Network, doi:10.6004/jnccn.2021.7309 | Real World Outcomes of Cancer Patients With SARS-CoV-2 Infection Receiving Monoclonal Antibodies |
| 51% lower hospitalization (p=0.06). Retrospective 395 patients in the USA receiving casirivimab/imdevimab or bamlanivimab, showing lower risk of hospitalization with treatment, statistically significant for casirivimab/imdevimab. | ||
Mar 12 2022 |
et al., medRxiv, doi:10.1101/2022.03.10.22272100 | Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19 |
| 12% faster recovery and 9% improved viral clearance (p<0.0001). RCT showing improved viral clearance with bamlanivimab/etesevimab combined with bebtelovimab. Results refer to the placebo controlled portion of the trial. | ||
Feb 16 2022 |
et al., bioRxiv, doi:10.1101/2022.02.15.480166 | SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies |
| In Vitro study showing that omicron BA.2 evades all monoclonal antibodies tested, including sotrovimab and tixagevimab/cilgavimab which retained activity for omicron BA.1. | ||
Feb 9 2022 |
et al., Journal of the American Geriatrics Society, doi:10.1111/jgs.17705 | Clinical Outcomes of Monoclonal Antibody Therapy During a COVID-19 Outbreak in a Skilled Nursing Facility - Arizona, 2021 |
| 89% lower mortality (p=0.01) and 86% lower progression (p=0.002). Retrospective 75 COVID+ patients in a skilled nursing facility in the USA, 56 treated within a median of 2 days from symptom onset with bamlanivimab, showing significantly lower mortality with treatment. | ||
Jan 27 2022 |
et al., Infectious Diseases in Clinical Practice, doi:10.1097/IPC.0000000000001109 | Impact of Rapidly Deployed COVID-19 Monoclonal Antibody Infusion Clinics on Rate of Hospitalization |
| 52% lower hospitalization (p=0.01) and 20% lower progression (p=0.52). Retrospective 438 patients in the USA, 253 treated with bamlanivimab, showing significantly lower hospitalization with treatment. | ||
Jan 27 2022 |
et al., Infectious Diseases in Clinical Practice, doi:10.1097/IPC.0000000000001130 | Bamlanivimab for the Prevention of Hospitalizations and Emergency Department Visits in SARS-CoV-2–Positive Patients in a Regional Health Care System |
| no change in mortality (p=1), 4% higher hospitalization (p=0.86), and 5% more combined hospitalization/ER visits (p=0.86). Retrospective 379 bamlanivimab patients and 379 matched controls in the USA, showing no significant differences with treatment. | ||
Dec 20 2021 |
et al., bioRxiv, doi:10.1101/2021.12.19.473354 | Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron) |
| In Vitro study showing that omicron is substantially resistant to neutralization by monoclonal antibodies REGN10933, REGN10987, Ly-CoV016 and Ly-CoV555. S309 (the parent of Sotrovimab) had only 2-fold loss in potency. | ||
Dec 17 2021 |
et al., bioRxiv, doi:10.1101/2021.12.15.472828 | An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies |
| In vitro study (Vero-TMPRSS2 and Vero-hACE2-TMPRSS2) showing complete loss of inhibitory activity for B.1.1.529 omicron with LY-CoV555, LY-CoV016, REGN10933, REGN10987, and CT-P59, ~12-fold decrease for COV2-2196/COV2-2130, and minimal ch.. | ||
Dec 15 2021 |
et al., bioRxiv, doi:10.1101/2021.12.14.472719 | Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2 |
| In Vitro study (Vero-E6-TMPRSS2) showing 18 of 19 monoclonal antibodies were no longer effective or significantly impaired with B.1.1.529 omicron. | ||
Nov 19 2021 |
et al., Cureus, doi:10.7759/cureus.19747 | Real World Utilization of Bamlanivimab at a Rural Community Hospital |
| 30% lower hospitalization (p=0.6) and 42% improvement (p=0.04). Retrospective 136 outpatients showing bamlanivimab reduced emergency department visits at 28 days, but not hospitalizations, compared to a control group prior to authoritzation in patients with mild to moderate COVID-19. | ||
Nov 3 2021 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab546 | Bamlanivimab efficacy in older and high BMI outpatients with Covid-19 selected for treatment in a lottery-based allocation process |
| 65% lower hospitalization (p=0.04). Retrospective database analysis of 1257 PCR+ outpatients with age ≥65, BMI≥35, 191 receiving bamlanivimab via lottery. Authors note that the alpha variant was most common during the study period, and that efficacy against other variants c.. | ||
Nov 2 2021 |
et al., Research Square, doi:10.21203/rs.3.rs-995033/v1 | Antibody escape and global spread of SARS-CoV-2 lineage A.27 |
| Anaysis of antibody escape showing variant A.27 completely escaped neutralization with LY-COV555 and partially with REGN10987. B.1.617.2 escaped these antibodies in a similar manner, suggesting that L452R facilitates the escape. Authors n.. | ||
Oct 29 2021 |
et al., Mediterranean Journal of Hematology and Infectious Diseases, doi:10.4084/MJHID.2021.061 | Early Treatment with BamlanivimabAlone does not Prevent COVID-19 Hospitalization and Its Post-Acute Sequelae. A Real Experience in Umbria, Italy |
| Retrospective 39 patients treated with bamlanivimab in Italy, showing results far below expectations based on the BLAZE1 trial. Authors note that most patients had the alpha variant which does not have the E484K and L452R mutations known .. | ||
Oct 8 2021 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab512 | Real-world Assessment of 2,879 COVID-19 Patients Treated with Monoclonal Antibody Therapy: A Propensity Score-Matched Cohort Study |
| 7% higher mortality (p=0.86), 17% lower ICU admission (p=0.51), and 24% lower hospitalization (p=0.0005). Retrospective 2,879 patients and matched controls in the USA, showing significantly lower mortality and hospitalization with monoclonal antibody treatment (bamlanivimab, bamlanivimab/etesevimab, or casirivimab/imdevimab). There was signif.. | ||
Oct 7 2021 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2102685 | Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19 |
| 95% lower mortality (p=0.002), 70% lower combined mortality/hospitalization (p=0.0002), 11% faster recovery (p=0.007), and 67% improved viral clearance (p<0.0001). Results from the BLAZE-1 RCT of combined bamlanivimab/etesevimab, showing significantly lower mortality and combined mortality/hospitalization with treatment. NCT04427501. | ||
Oct 1 2021 |
et al., Journal of Clinical Investigation, doi:10.1172/JCI151697 | Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19 |
| 74% lower mortality (p=0.11), 49% lower ICU admission (p=0.1), and 37% lower hospitalization (p=0.01). Retrospective 2,335 bamlanivimab patients and 2,335 PSM controls in the USA, showing significantly lower hospitalization with treatment. | ||
Sep 15 2021 |
et al., medRxiv, doi:10.1101/2021.09.03.21263105 | Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy |
| Analysis of ACTIV-2/A5401, showing the potential for rapid emergence of resistance during monoclonal antibody treatment, resulting in prolonged high level viral loads and clinical worsening. Treatment-emergent resistance mutations were mo.. | ||
Jun 23 2021 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab331 | Real-World Effectiveness and Tolerability of Monoclonal Antibody Therapy for Ambulatory Patients with Early COVID-19 |
| 80% lower mortality (p=0.09), 53% lower hospitalization (p<0.0001), and 27% fewer combined hospitalization/ER visits (p<0.0001). Retrospective 479 patients treated with bamlanivimab showing lower mortality, hospital admission, and emergency department visits with treatment. Authors incorrectly state that "no other COVID-19 therapies for ambulatory patients hav.. | ||
Jun 10 2021 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab305 | The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting |
| 80% lower mortality (p=0.08) and 39% lower hospitalization (p=0.0004). Retrospective 780 bamlanivimab patients and 5,337 patients not receiving treatment, showing lower hospitalization and ER visits with treatment. | ||
May 18 2021 |
et al., Research Square, doi:10.21203/rs.3.rs-524959/v1 | Emergence of SARS-CoV-2 Spike Escape Mutation Q493r After Bamlanivimab/Etesevimab Treatment for COVID-19 |
| Case study showing that a mutation resistant to both bamlanivimab and etesevimab can arise in vivo. Authors note that accelerated evolution can occur under selective pressure from therapeutic interventions with neutralizing antibodies, an.. | ||
May 16 2021 |
et al., Military Medicine, doi:10.1093/milmed/usab188 | Bamlanivimab Use in a Military Treatment Facility |
| 62% fewer combined hospitalization/ER visits (p=0.22). Retrospective 40 outpatients showing improvement in symptoms and lower risk of hospitalization/ER visits with bamlanivimab, without statistical significance. Different counts for hospitalization are provided in the abstract and text: &quo.. | ||
May 10 2021 |
et al., Cureus, doi:10.7759/cureus.14933 | Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study |
| 75% lower mortality (p=0.03) and 65% lower hospitalization (p=0.08). Retrospective 246 nursing home patients showing lower mortality with early bamlanivimab treatment. | ||
Mar 30 2021 |
et al., medRxiv, doi:10.1101/2021.03.25.21254322 | Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection |
| 67% lower mortality (p=0.05), 64% lower combined mortality/hospitalization (p=0.0003), and 61% lower hospitalization (p=0.001). Retrospective 234 patients receiving bamlanivimab and 234 matched controls, showing lower hospitalization and mortality with treatment. Greater benefit was seen with administration within 4 days of their positive COVID-19 test. | ||
Jan 21 2021 |
, Press Release | Lilly's neutralizing antibody bamlanivimab (LY-CoV555) prevented COVID-19 at nursing homes in the BLAZE-2 trial, reducing risk by up to 80 percent for residents |
| 57% fewer symptomatic cases (p=0.0002). Press release on the BLAZE-2 trial at nursing homes showing significantly lower symptomatic COVID-19 with treatment. | ||
Jan 21 2021 |
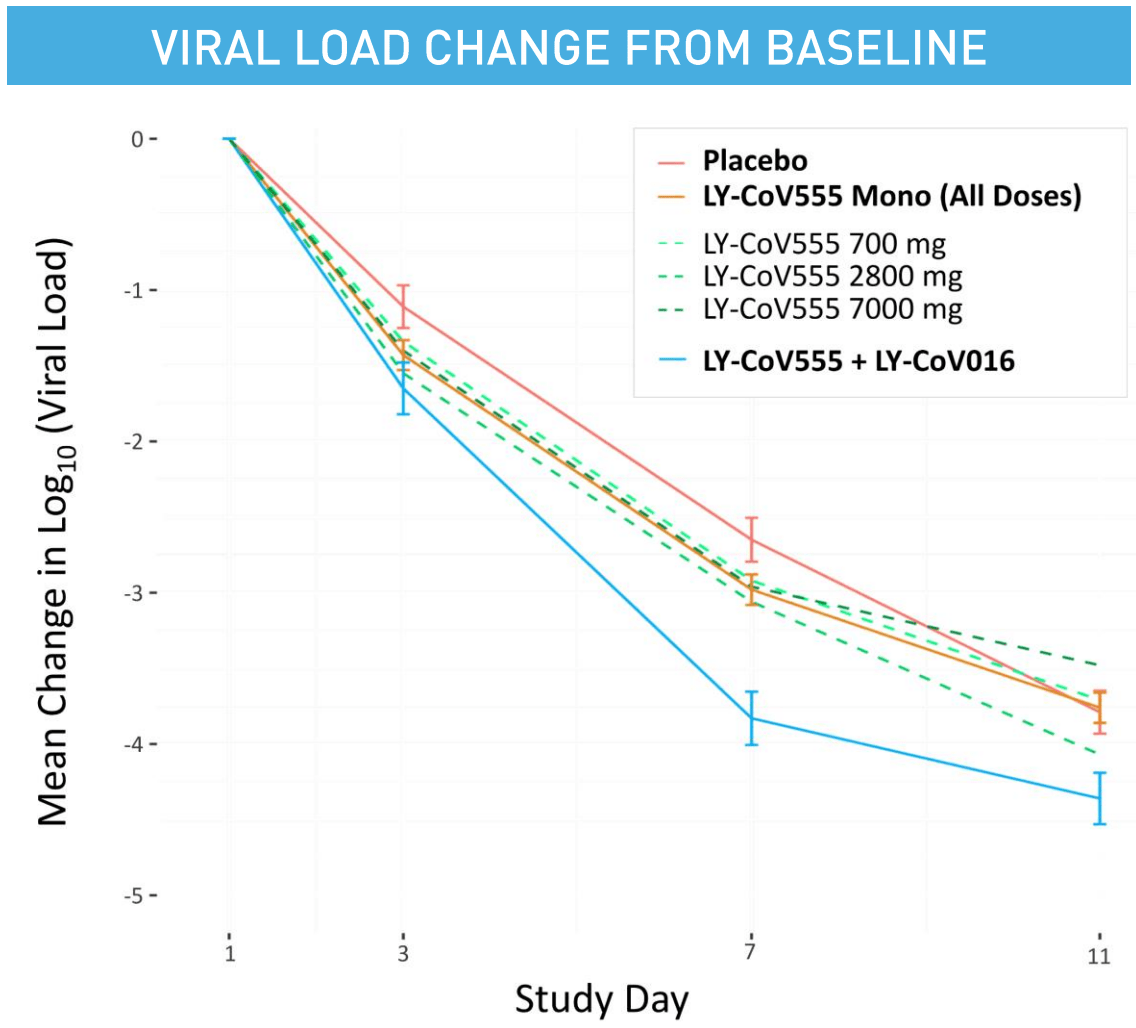
et al., JAMA, doi:10.1001/jama.2021.0202 | Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19 |
| 71% fewer combined hospitalization/ER visits (p=0.05). RCT for LY-CoV555 monotherapy and LY-CoV555/LY-CoV016 combination therapy with 592 patients showing lower hospitalization/ER visits with treatment. For viral load at day 11, a statistically significant reduction was found with combination.. | ||
Dec 22 2020 |
, NEJM, doi:10.1056/NEJMoa2033130 | A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19 |
| 100% higher mortality (p=0.22). Late stage RCT of LY-CoV555 added to remdesivir, showing non-statistically significant higher mortality with the addition of LY-CoV555. | ||
Oct 28 2020 |
et al., NEJM, doi:10.1056/NEJMoa2029849 | SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19 |
| 74% lower hospitalization (p=0.02). Interim analysis of the BLAZE-1 phase 2 trial of outpatients showing lower hospitalization or ER visits (1.6% versus 6.3%), and improvements in symptoms and viral load compared to placebo. NCT04427501. | ||
Oct 26 2020 |
, Press Release | Lilly Statement Regarding NIH’s ACTIV-3 Clinical Trial |
| ACTIV-3 intermin analysis shows LY-CoV555 is unlikely to help hospitalized patients. ACTIV-2, BLAZE-1 and BLAZE-2 trials remain ongoing. | ||
Oct 7 2020 |
, Press Release | SARS-CoV-2 neutralizing antibody program update |
| 84% fewer combined hospitalization/ER visits (p=0.05). Interim results from the BLAZE-1 outpatient RCT showing improvements in viral load, symptoms and hospitalization. Combination therapy significantly reduced viral load at day 11 (p=0.011). A greater effect is seen at day 7 (p<0.001). The p.. | ||
Please send us corrections, updates, or comments.
c19early involves the extraction of 100,000+ datapoints from
thousands of papers. Community updates
help ensure high accuracy.
Treatments and other interventions are complementary.
All practical, effective, and safe
means should be used based on risk/benefit analysis.
No treatment or intervention is 100% available and effective for all current
and future variants.
We do not provide medical advice. Before taking any medication,
consult a qualified physician who can provide personalized advice and details
of risks and benefits based on your medical history and situation. FLCCC and WCH
provide treatment protocols.
Thanks for your feedback! Please search before submitting papers and note
that studies are listed under the date they were first available, which may be
the date of an earlier preprint.