May 4 |
Remdesivir for COVID-19: real-time meta analysis of 70 studies | |
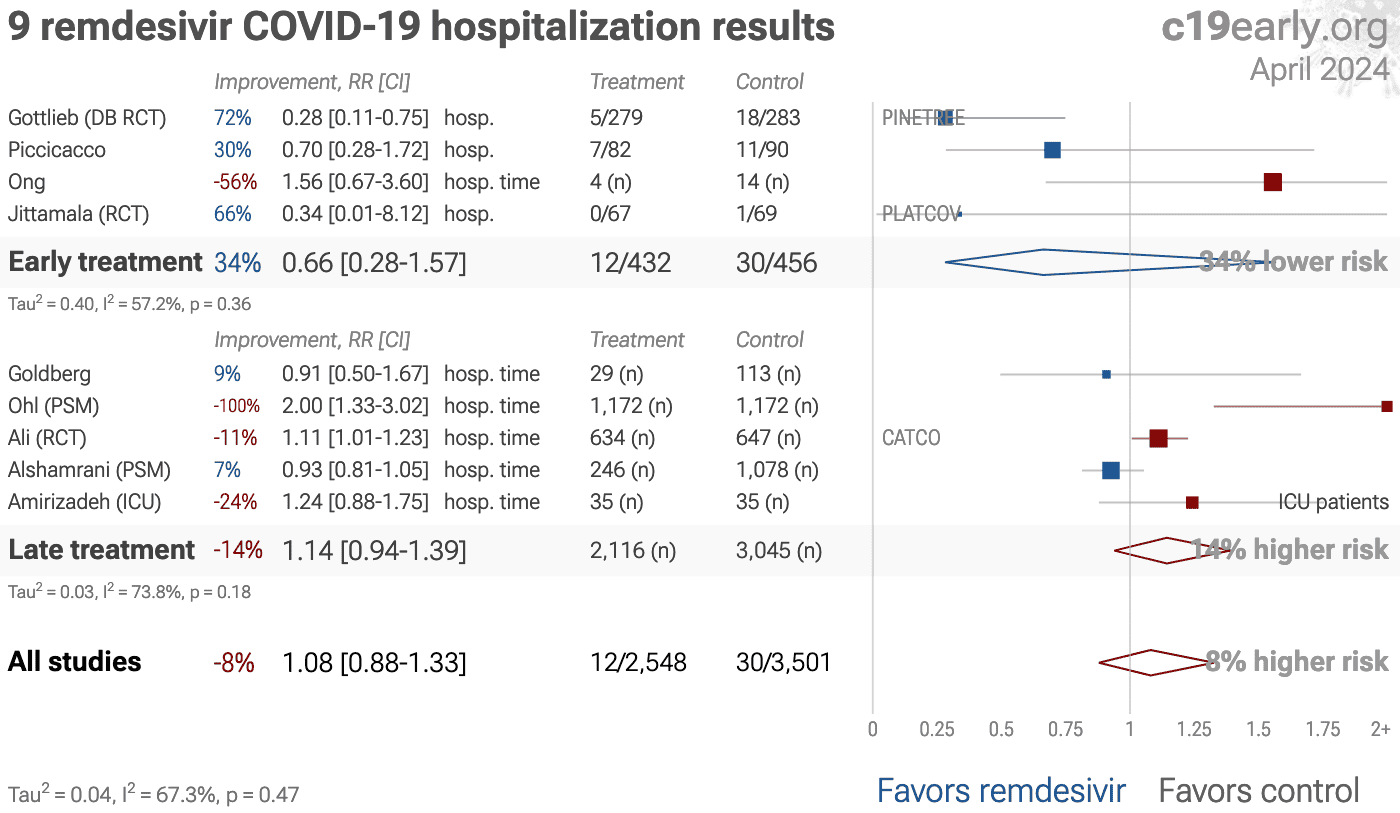
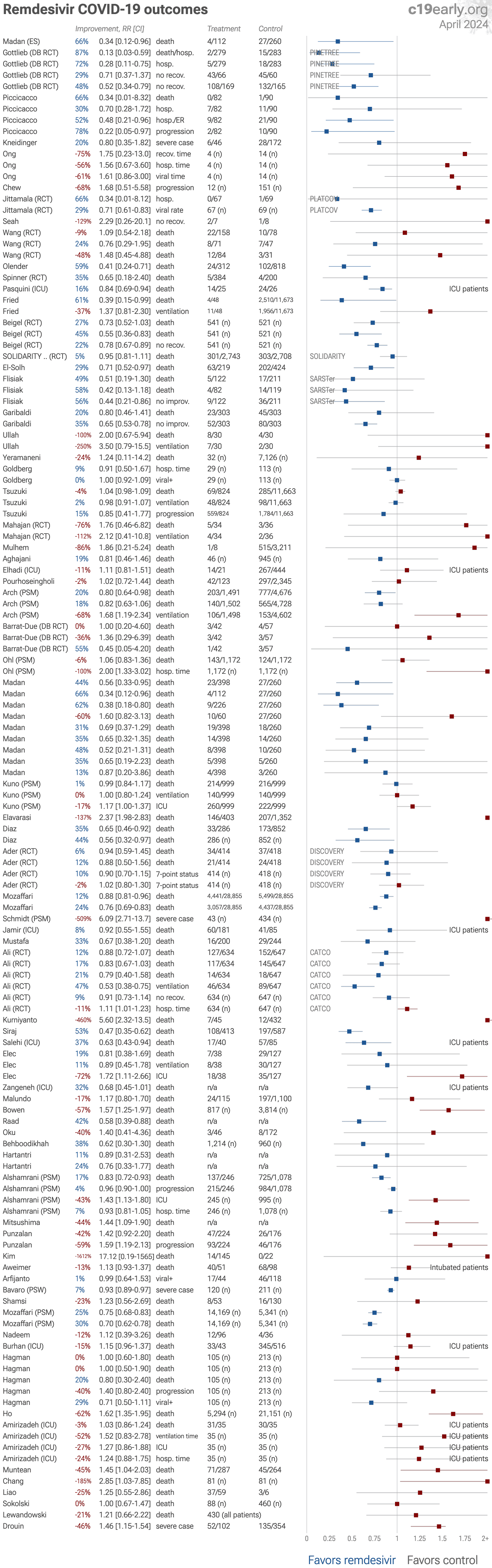
| Meta analysis shows 4% [-5‑12%] lower mortality, and pooled analysis using the most serious outcome reported shows 2% [-6‑9%] lower risk, without reaching statistical significance. While studies to date show a small .. | ||
Apr 4 |
et al., Scientific Reports, doi:10.1038/s41598-024-57726-3 | The metabolic footprint of Vero E6 cells highlights the key metabolic routes associated with SARS-CoV-2 infection and response to drug combinations |
| In Vitro study showing that remdesivir and azithromycin, either alone or in combination, can modify the glycolic-gluconeogenesis pathway in host cells, inhibiting the mitochondrial oxidative damage caused by SARS-CoV-2. Authors use Nuclea.. | ||
Apr 3 |
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01015-23 | Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents |
| In Silico study supporting the synergistic combination of nitazoxanide and remdesivir for SARS-CoV-2. Authors developed a two-way pharmacodynamic modeling approach to capture the concentration-dependent drug-drug interactions and combined.. | ||
Mar 19 |
et al., Scientific Reports, doi:10.1038/s41598-024-57306-5 | Clinical and laboratory characteristics of patients hospitalized with severe COVID-19 in New Orleans, August 2020 to September 2021 |
| 46% higher severe cases (p<0.0001). Retrospective 456 hospitalized patients in the USA showing an association between remdesivir treatment and increased COVID-19 severity in multivariable analysis, for remdesivir treatment within 7 days and when administered before meeting .. | ||
Mar 7 |
et al., Biomedicines, doi:10.3390/biomedicines12030605 | Insulin and Metformin Administration: Unravelling the Multifaceted Association with Mortality across Various Clinical Settings Considering Type 2 Diabetes Mellitus and COVID-19 |
| 21% higher mortality (p=0.55). Retrospective 430 hospitalized COVID-19 patients with type 2 diabetes in Poland showing lower mortality with metformin and higher mortality with remdesivir, convalescent plasma, and aspirin in univariable analysis. These results were not.. | ||
Feb 28 |
et al., Scientific Reports, doi:10.1038/s41598-024-55407-9 | Antiplatelet therapy prior to COVID-19 infection impacts on patients mortality: a propensity score-matched cohort study |
| no change in mortality (p=1). Retrospective 2,170 hospitalized COVID-19 patients showing no difference in mortality with remdesivir in unadjusted results. | ||
Feb 6 |
et al., Research Square, doi:10.21203/rs.3.rs-3899998/v1 | Real-world Efficacy of Ensitrelvir in Hospitalized Patients with COVID-19 in Japan: A Retrospective Observational Study |
| Retrospective 154 hospitalized COVID-19 patients in Japan showing faster viral clearance and shorter hospitalization with ensitrelvir treatment compared to remdesivir or molnupiravir. There was no significant difference for fever resoluti.. | ||
Jan 26 |
et al., JAMA Health Forum, doi:10.1001/jamahealthforum.2023.5044 | Clinical Risk and Outpatient Therapy Utilization for COVID-19 in the Medicare Population |
| Analysis of Medicare beneficiaries in 2022 showing that outpatient COVID-19 treatments like antivirals and monoclonal antibodies were disproportionately used by patients at lower risk of severe infection and outcomes. Retrospective studie.. | ||
Jan 15 |
et al., BMC Pulmonary Medicine, doi:10.1186/s12890-024-02850-z | Clinical characteristics and outcomes among critically ill patients with cancer and COVID-19-related acute respiratory failure |
| 25% higher mortality (p=0.67). Retrospective study of 215 critically ill COVID-19 patients with respiratory failure showing higher mortality for cancer patients. Remdesivir was used more for non-survivors, without statistical significance. Most patients received remdes.. | ||
Dec 29 2023 |
et al., Medicine, doi:10.1097/MD.0000000000036777 | The association between COVID-19 vaccination and confirmed patients with hospitalization in Omicron era: A retrospective study |
| 185% higher mortality (p=0.04). Retrospective 209 hospitalized COVID-19 patients in Taiwan showing higher mortality with a 5-day course of remdesivir compared to other antivirals or no antiviral treatment in multivariable analysis. Adjustments include qSOFA and CCI, wit.. | ||
Dec 19 2023 |
et al., Pharmaceuticals, doi:10.3390/ph17010003 | Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre |
| 45% higher mortality (p=0.03). Retrospective 551 severe/critical COVID-19 patients showing higher mortality and higher risk of drug induced liver injury with remdesivir. Authors appear to have reversed the OR for remdesivir - use was more common in non-survivors (61% v.. | ||
Dec 14 2023 |
et al., Pathogens, doi:10.3390/pathogens12121453 | Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting |
| Retrospective 9,049 hospitalized COVID-19 patients in Romania showing increased risk of acute kidney injury and liver injury with remdesivir treatment. | ||
Dec 14 2023 |
et al., Health Science Reports, doi:10.1002/hsr2.1698 | Remdesivir therapy for severe pediatric COVID‐19 in Singapore: A single‐center retrospective observational cohort study |
| 129% worse recovery (p=0.57). Retrospective 15 pediatric patients hospitalized for severe COVID-19 requiring oxygen and high dependency/intensive care unit (HD/ICU) admission in Singapore, showing no improvement in deescalation from HD/ICU care with remdesivir, howeve.. | ||
Dec 6 2023 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2023.1287542 | Unveiling patenting strategies of therapeutics and vaccines: evergreening in the context of COVID-19 pandemic |
| Review of the patenting activity and evergreening approaches for three major COVID-19 antiviral medications – remdesivir, molnupiravir, and favipiravir. Authors found extensive primary and secondary patent filing, with 29 applications cov.. | ||
Nov 21 2023 |
et al., The Journal of Tehran University Heart Center, doi:10.18502/jthc.v18i3.14114 | The Association between Acute Cardiac Injury and Outcomes of Hospitalized Patients with COVID-19: Long-term Follow-up Results from the Sina Hospital COVID-19 Registry, Iran |
| Retrospective 1,413 hospitalized COVID-19 patients evaluating the association between acute cardiac injury (ACI) and outcomes. Authors include a multivariable analysis showing lower risk of ACI with HCQ and higher risk with remdesivir, wi.. | ||
Nov 1 2023 |
et al., Health Science Reports, doi:10.1002/hsr2.1676 | The effect of remdesivir on mortality and the outcome of patients with COVID‐19 in intensive care unit: A case–control study |
| 3% higher mortality (p=1), 52% longer ventilation (p=0.17), 27% longer ICU admission (p=0.23), and 24% longer hospitalization (p=0.22). Retrospective 70 COVID-19 ICU patients, 35 receiving remdesivir plus standard treatment and 35 receiving standard treatment only. No significant differences were found for mortality, hospitalization time, ICU time, or ventilation time. | ||
Oct 31 2023 |
et al., HCA Healthcare Journal of Medicine, doi:10.36518/2689-0216.1546 | A Retrospective Cohort Study Assessing the Impact of Statin Therapy on Hospital Length of Stay and Inpatient Mortality in COVID-19 Patients |
| 62% higher mortality (p<0.0001). Retrospective 26,445 hospitalized COVID-19 patients in the USA, showing higher mortality with remdesivir. | ||
Sep 28 2023 |
et al., Vaccines, doi:10.3390/vaccines11101533 | In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 |
| In Vitro study showing sharply reduced neutralization of SARS-CoV-2 variants XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 with monoclonal antibodies cilgavimab, tixagevimab, imdevimab, etsevimab, casirivim.. | ||
Sep 26 2023 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkad295 | Effects of remdesivir on SARS-CoV-2 viral dynamics and mortality in viraemic patients hospitalized for COVID-19 |
| no change in mortality (p=0.97), 40% higher progression (p=0.31), and 29% improved viral clearance (p=0.11). Retrospective 318 hospitalized COVID-19 patients in Sweden, showing improvements in viral clearance but no improvement for mortality with remdesivir treatment. | ||
Sep 25 2023 |
et al., PLOS ONE, doi:10.1371/journal.pone.0290964 | Characteristics and outcomes of patients with severe COVID-19 in Indonesia: Lessons from the first wave |
| 15% higher mortality (p=0.23). Retrospective 559 COVID-19 ICU patients in Indonesia, showing higher mortality with remdesivir in unadjusted results, without statistical significance. Note that confounding by indication should be less significant for ICU studies compare.. | ||
Sep 16 2023 |
et al., Natural Product Communications, doi:10.1177/1934578X231188861 | In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds |
| In vitro study showing that of 9 phenolic compounds tested, only curcumin inhibited SARS-CoV-2 cytopathic effects in infected monkey kidney Vero E6 cells. Curcumin showed antiviral activity against wildtype, alpha, delta, and omicron vari.. | ||
Aug 12 2023 |
et al., Cureus, doi:10.7759/cureus.43389 | Effects of Different Anticoagulation Doses on Moderate-to-Severe COVID-19 Pneumonia With Hypoxemia |
| 12% higher mortality (p=1). Retrospective 132 hospitalized COVID-19 patients in the USA, showing no significant difference in mortality with remdesivir in unadjusted results. | ||
Aug 9 2023 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciad460 | Remdesivir reduced mortality in immunocompromised patients hospitalized for COVID-19 across variant waves: Findings from routine clinical practice. |
| 25% lower mortality (p<0.0001). Retrospective 19,184 immunocompromised patients treated with remdesivir and matched controls, showing lower mortality with treatment. Several authors work at Gilead and the study was funded by Gilead. The majority of patients were treated.. | ||
Jul 20 2023 |
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiad275 | Clinical antiviral efficacy of remdesivir in COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV) |
| 29% improved viral clearance (p<0.0001). High conflict of interest RCT with very low risk patients with high existing immunity, showing faster viral clearance with remdesivir. The viral clearance half-life was very short in both arms. With rapid viral clearance and very low risk.. | ||
Jul 17 2023 |
et al., Canadian Journal of Infectious Diseases and Medical Microbiology, doi:10.1155/2023/5205188 | Survival and Mortality in Hospitalized Children with COVID-19: A Referral Center Experience in Yazd, Iran |
| 23% higher mortality (p=0.63). Retrospective 183 hospitalized pediatric COVID-19 patients in Iran, showing no significant difference in mortality with in unadjusted results. | ||
Jun 14 2023 |
et al., Journal of Korean Medical Science, doi:10.3346/jkms.2023.38.e217 | Clinical Characteristics and Risk Factors for Mortality in Critical COVID-19 Patients Aged 50 Years or Younger During Omicron Wave in Korea: Comparison With Patients Older Than 50 Years of Age |
| Retrospective 213 critical COVID-19 patients in South Korea, reporting no significant difference in mortality with remdesivir treatment (results not provided). | ||
Jun 10 2023 |
et al., Value in Health, doi:10.1016/j.jval.2023.03.2056 | Variation in Demographic Characteristics, Socioeconomic Status, Clinical Presentation and Selected Treatments in Mortality Among Patients with a Diagnosis of COVID-19 in the United States |
| Retrospective analysis of mortality for COVID-19 patients in the USA. Authors do not provide adjusted results, preventing any strong evidence. However it is notable that, despite comparable treatment frequencies, the mortality for patient.. | ||
Jun 1 2023 |
et al., Stem Cell Reports, doi:10.1016/j.stemcr.2023.05.007 | Parallel use of human stem cell lung and heart models provide insights for SARS-CoV-2 treatment |
| In Vitro study showing that SARS-CoV-2 cell entry differs across cell types. ACE2 was required for infection in both lung and cardiac cells, but TMPRSS2 cleavage was required in lung cells, while the endosomal pathway was required in card.. | ||
May 19 2023 |
et al., Viruses, doi:10.3390/v15051199 | Efficacy of Remdesivir and Neutralizing Monoclonal Antibodies in Monotherapy or Combination Therapy in Reducing the Risk of Disease Progression in Elderly or Immunocompromised Hosts Hospitalized for COVID-19: A Single Center Retrospective Study |
| 7% lower severe cases (p=0.001). Retrospective 331 hospitalized COVID-19 patients in Italy, showing lower progression with remdesivir. Combination therapy with mAbs was more effective, and improved results were seen for immunocompromised patients. | ||
May 4 2023 |
et al., Pathophysiology, doi:10.3390/pathophysiology30020016 | Duration of SARS-CoV-2 RNA Shedding Is Significantly Influenced by Disease Severity, Bilateral Pulmonary Infiltrates, Antibiotic Treatment, and Diabetic Status: Consideration for Isolation Period |
| 1% improved viral clearance (p=1). Retrospective 162 hospitalized COVID-19 patients in Indonesia, showing no significant difference in delayed viral clearance with remdesivir treatment in unadjusted results. | ||
Mar 29 2023 |
et al., Scientific Reports, doi:10.1038/s41598-023-31944-7 | Mortality rates of severe COVID-19-related respiratory failure with and without extracorporeal membrane oxygenation in the Middle Ruhr Region of Germany |
| 13% higher mortality (p=0.33). Retrospective 149 patients under invasive mechanical ventilation in Germany showing no significant difference in mortality with remdesivir in unadjusted results. | ||
Mar 16 2023 |
et al., Pathogens, doi:10.3390/pathogens12030473 | Clinical Predictors for Abnormal ALT in Patients Infected with COVID-19—A Retrospective Single Centre Study |
| 68% higher progression (p=0.4). Retrospective 163 COVID-19 patients in Singapore, showing increased risk of liver injury (abnormal ALT) with acetaminophen in a dose-dependent manner, and with remdesivir, without statistical significance in both cases. | ||
Mar 15 2023 |
et al., Journal of Clinical Medicine, doi:10.3390/jcm12062279 | Clinical Outcome and Prognosis of a Nosocomial Outbreak of COVID-19 |
| 1612% higher mortality (p=0.22). Retrospective 167 nosocomial COVID-19 patients in South Korea, showing higher mortality with remdesivir treatment, without statistical significance. | ||
Feb 28 2023 |
et al., Frontiers in Immunology, doi:10.3389/fimmu.2023.1123497 | Utility of laboratory and immune biomarkers in predicting disease progression and mortality among patients with moderate to severe COVID-19 disease at a Philippine tertiary hospital |
| 42% higher mortality (p=0.12) and 59% higher progression (p=0.001). Prospective study of 400 hospitalized patients in the Philippines, showing higher progression with remdesivir in unadjusted results, without statistical significance. | ||
Feb 21 2023 |
et al., International Journal of General Medicine, doi:10.2147/IJGM.S394413 | Risk of Underlying Diseases and Effectiveness of Drugs on COVID-19 Inpatients Assessed Using Medical Claims in Japan: Retrospective Observational Study |
| 44% higher mortality (p=0.01). Retrospective 18,566 hospitalized patients in Japan, showing higher mortality with remdesivir treatment. | ||
Feb 15 2023 |
et al., Saudi Pharmaceutical Journal, doi:10.1016/j.jsps.2023.02.004 | Comprehensive evaluation of six interventions for hospitalized patients with COVID-19: A propensity score matching study |
| 17% lower mortality (p=0.003), 4% lower progression (p=0.12), 43% longer ICU admission (p=0.003), and 7% shorter hospitalization (p=0.25). PSM retrospective 29 hospitals in Saudi Arabia, showing lower mortality with remdesivir treatment. | ||
Feb 13 2023 |
et al., Advances in Animal and Veterinary Sciences, doi:10.17582/journal.aavs/2023/11.3.404.409 | The Potential of Melatonin and N-Acetylcysteine on Remdesivir Induced Liver Injury in Covid 19 Patients |
| RCT 70 hospitalized COVID-19 patients evaluating the effects of N-acetylcysteine (NAC) and melatonin on liver injury induced by remdesivir treatment. NAC 600mg twice daily and melatonin 6mg daily. All patients received remdesivir. Liver e.. | ||
Feb 9 2023 |
et al., The Lancet Regional Health - Southeast Asia, doi:10.1016/j.lansea.2023.100167 | Clinical and treatment factors associated with the mortality of COVID-19 patients admitted to a referral hospital in Indonesia |
| 11% lower mortality (p=0.84). Retrospective 689 hospitalized patients in Indonesia, showing no significant difference in mortality with remdesivir treatment. | ||
Jan 20 2023 |
et al., Acta Oncologica, doi:10.1080/0284186X.2023.2169079 | A cohort study of COVID-19 infection in pediatric oncology patients plus the utility and safety of remdesivir treatment |
| 75% slower recovery (p=0.6), 56% longer hospitalization (p=0.31), and 61% slower viral clearance (p=0.14). Retrospective 18 immunocompromised pediatric COVID-19 patients in Singapore, showing slower viral clearance with remdesivir, without statistical significance. | ||
Nov 10 2022 |
et al., PLoS ONE, doi:10.1371/journal.pone.0276751 | Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients |
| Vero E6 In Vitro study showing remdesivir and ivermectin or azithromycin to be highly synergistic with 4-13 times lower concentration required for 100% inhibition. | ||
Sep 15 2022 |
et al., Iranian Journal of Science and Technology, Transactions A: Science, doi:10.1007/s40995-022-01351-0 | Evaluation of the Costs and Outcomes of COVID-19 Therapeutic Regimens in Hospitalized Patients in Shiraz |
| 38% lower mortality (p=0.21). Retrospective 2,174 hospitalized patients showing no significant differences with remdesivir treatment. | ||
Sep 9 2022 |
et al., Infection, doi:10.1007/s15010-022-01914-8 | Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study |
| 20% lower severe cases (p=0.71). Retrospective 218 COVID+ lung transplant patients in Germany, showing no significant difference in severe cases with early remdesivir use. | ||
Sep 6 2022 |
et al., Modern Rheumatology, doi:10.1093/mr/roac104 | Risk factors for hospitalization or mortality for COVID-19 in patients with rheumatic diseases: Results of a nation-wide JCR COVID-19 registry in Japan |
| 40% higher mortality (p=0.59). Retrospective 220 COVID-19 patients with rheumatic disease in Japan, showing no significant difference in mortality with remdesivir treatment. | ||
Aug 26 2022 |
et al., medRxiv, doi:10.1101/2022.08.25.22279181 | International Multicenter Study Comparing Cancer to Non-Cancer Patients with COVID-19: Impact of Risk Factors and Treatment Modalities on Survivorship |
| 42% lower mortality (p=0.009). Retrospective 3,966 COVID-19 patients, 1,115 with cancer, showing lower mortality with remdesivir and higher mortality with convalescent plasma. | ||
Aug 25 2022 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac436 | Reduction in risk of death among patients admitted with COVID-19 between first and second epidemic waves in New York City |
| 57% higher mortality (p=0.0001). Retrospective 4,631 hospitalized patients in New York, showing higher mortality with remdesivir, and lower mortality with HCQ. Authors suggest that increased mortality during the first epidemic wave was partly due to strain on hospital re.. | ||
Aug 1 2022 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac256 | Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge |
| 30% lower hospitalization (p=0.47), 52% fewer combined hospitalization/ER visits (p=0.05), and 78% lower progression (p=0.03). Retrospective high-risk outpatients in the USA, 82 treated with remdesivir, 88 with sotrovimab, and 90 control patients, showing significantly lower combined hospitalization/ER visits with both treatments in unadjusted results. The domina.. | ||
Jul 14 2022 |
et al., IJID Regions, doi:10.1016/j.ijregi.2022.07.009 | Predictors of Mortality among inpatients with COVID-19 Infection in a Tertiary Referral Center in the Philippines |
| 17% higher mortality (p=0.45). Retrospective 1,215 hospitalized patients in the Phillipines, showing no significant difference in outcomes with remdesivir or HCQ use in unadjusted results subject to confounding by indication. | ||
May 31 2022 |
et al., Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014 | Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points |
| 416% higher progression (p=0.04). Retrospective 292 patients in Switzerland showing liver damage associated with acetaminophen treatment and remdesivir treatment. | ||
May 13 2022 |
et al., Obesity Medicine, doi:10.1016/j.obmed.2022.100420 | Survival analysis based on body mass index in patients with Covid-19 admitted to the intensive care unit of Amir Al-Momenin Hospital in Arak – 2021 |
| 32% lower mortality (p=0.06). Retrospective 193 ICU patients in Iran, showing lower mortality with remdesivir treatment, not reaching statistical significance. | ||
Apr 2 2022 |
et al., Pharmaceuticals, doi:10.3390/ph15040445 | Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants |
| In Vitro study with 30 COVID-19 strains from 14 variants, showing stronger efficacy with ivermectin compared to CQ and remdesivir, and relatively homogeneous efficacy with ivermectin regardless of strain/variant, in contrast to results fo.. | ||
Mar 25 2022 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828 | Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS |
| FAERS disproportionality analysis identifying a significant association between remdesivir and AKI, PSM ROR 3.85 [3.11-4.78]. | ||
Mar 17 2022 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679 | Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis |
| FAERS analysis showing significantly increased risk of acute kidney injury with remdesivir. | ||
Mar 14 2022 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.03.015 | COVID-19 and Kidney Transplantation: The impact of remdesivir on renal function and outcome - a retrospective cohort study |
| 19% lower mortality (p=0.66), 11% lower ventilation (p=0.73), and 72% higher ICU admission (p=0.01). Retrospective 165 hospitalized COVID-19+ kidney transplant patients, 38 treated with remdesivir, showing no significant difference in mortality, higher ICU admission, and lower ICU mortality. Subject to confounding by time with significan.. | ||
Mar 11 2022 |
et al., Research Square, doi:10.21203/rs.3.rs-1362678/v1 | Risk factors of death in mechanically ventilated COVID-19 patients: a retrospective multi-center study |
| 37% lower mortality (p=0.01). Retrospective 125 mechanically ventilated ICU patients in Iran, showing lower mortality with remdesivir treatment in unadjusted results. | ||
Feb 28 2022 |
et al., Indian Journal of Clinical Practice, 32:9 | Efficacy of Various Treatment Modalities on Patient-related Outcome in Hospitalized COVID-19 Patients – A Retrospective Study |
| 53% lower mortality (p<0.0001). Retrospective 1,000 COVID+ hospitalized patients in India, showing lower mortality with famotidine and remdesivir in multivariable logistic regression. | ||
Feb 28 2022 |
et al., Journal of Clinical Virology Plus, doi:10.1016/j.jcvp.2022.100068 | Factors Associated with Death and ICU Referral among COVID-19 Patients Hospitalized in the Secondary Referral Academic Hospital in East Jakarta, Indonesia |
| 460% higher mortality (p=0.0009). Retrospective 477 hospitalized patients in Indonesia, showing higher mortality with remdesivir in unadjusted results. | ||
Jan 19 2022 |
et al., Canadian Medical Association Journal, doi:10.1503/cmaj.211698 | Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial |
| 12% lower mortality (p=0.21), 47% lower ventilation (p=0.0003), 9% improved recovery (p=0.41), and 11% longer hospitalization (p=0.04). RCT 1,282 hospitalized patients in Canada showing lower mechanical ventilation with remdesivir treatment, but no significant difference for mortality. | ||
Dec 30 2021 |
et al., Science Translational Medicine, doi:10.1126/scitranslmed.abl8282 | Inhaled remdesivir reduces viral burden in a nonhuman primate model of SARS-CoV-2 infection |
| African green monkey study of inhaled versus IV remdesivir, showing similar efficacy with inhalation. Comparable concentrations of the active triphosphate in the lower respiratory tract were found with ~20x lower dose using inhalation, an.. | ||
Dec 29 2021 |
et al., Exploratory Research in Clinical and Social Pharmacy, doi:10.1016/j.rcsop.2021.100101 | Pattern of medication utilization in hospitalized patients with COVID-19 in three District Headquarters Hospitals in the Punjab province of Pakistan |
| 33% lower mortality (p=0.21). Retrospective 444 hospitalized patients in Pakistan, showing lower mortality with remdesivir treatment in unadjusted results, not reaching statistical significance. | ||
Dec 22 2021 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2116846 | Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients |
| 87% lower combined mortality/hospitalization (p=0.008), 72% lower hospitalization (p=0.009), and 29% improved recovery (p=0.31). RCT high-risk outpatients, 279 treated with remdesivir and 283 control patients, median 5 days from symptoms, showing significantly lower hospitalization with treatment. | ||
Dec 13 2021 |
et al., Cureus, doi:10.7759/cureus.20394 | Determinants of Outcome Among Critically Ill Police Personnel With COVID-19: A Retrospective Observational Study From Andhra Pradesh, India |
| 8% lower mortality (p=0.77). Retrospective 266 COVID-19 ICU patients in India, showing significantly lower mortality with PVP-I oral gargling and topical nasal use, and non-statistically significant higher mortality with ivermectin and lower mortality with remdesivir. | ||
Nov 12 2021 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.34330 | Association Between Androgen Deprivation Therapy and Mortality Among Patients With Prostate Cancer and COVID-19 |
| 509% higher severe cases (p<0.0001). Retrospective 1,106 prostate cancer patients, showing higher mortality with remdesivir treatment. | ||
Oct 1 2021 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciab875 | Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort |
| 12% lower mortality (p=0.003). Retrospective 28,855 remdesivir patients with PSM matched controls, showing lower mortality with treatment. | ||
Sep 14 2021 |
et al., Lancet Infectious Diseases, doi:10.1016/S1473-3099(21)00485-0 | Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial |
| 6% lower mortality (p=0.77) and 10% improved 7-point scale results (p=0.39). RCT 857 hospitalized patients, showing no significant differences with remdesivir treatment. EudraCT2020-000936-23. | ||
Sep 10 2021 |
et al., The American Journal of Tropical Medicine and Hygiene, doi:10.4269/ajtmh.21-0606 | Remdesivir Efficacy in COVID-19 Treatment: A Randomized Controlled Trial |
| This paper was retracted. | ||
Aug 19 2021 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciab698 | Remdesivir and Mortality in Patients with COVID-19 |
| 35% lower mortality (p=0.01). Retrospective 1138 hospitalized patients in the USA, 286 treated with remdesivir, showing lower mortality with treatment. Age was not included in the adjustments (authors excluded variables that contributed to another score, in this case .. | ||
Aug 16 2021 |
et al., Microbes and Infectious Diseases, doi:10.21608/mid.2021.85877.1177 | Comparative study between the therapeutic effect of remdesivir versus hydroxychloroquine in COVID-19 hospitalized patients |
| Small study comparing 25 HCQ and 25 remdesivir hospitalized patients, reporting faster viral clearance with remdesivir. The article proof is missing the results for the HCQ group. Confounding by time is likely - remdesivir patients were a.. | ||
Aug 13 2021 |
et al., medRxiv, doi:10.1101/2021.08.13.21261992 | Another step toward final call on Remdesivir efficacy as a treatment for hospitalized COVID-19 patients: a multicenter open-label trial |
| Single arm remdesivir trial with 145 hospitalized patients showing no statistically significant difference between "early" and "late" administration, however the treatment delays may be better described as late and ver.. | ||
Aug 12 2021 |
et al., Lung India, doi:10.4103/lungindia.lungindia_493_21 | Clinical features, demography, and predictors of outcomes of SARS-CoV-2 infection at a tertiary care hospital in India: A cohort study |
| 137% higher mortality (p<0.0001). Retrospective 2017 hospitalized patients in India, showing higher mortality with remdesivir in unadjusted results, however no group details are provided and this result is subject to confounding by indication, with authors suggesting trea.. | ||
Aug 9 2021 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkab256 | The association of remdesivir and in-hospital outcomes for COVID-19 patients treated with steroids |
| 1% lower mortality (p=0.96), no change in ventilation (p=1), and 17% higher ICU admission (p=0.05). PSM retrospective 3,372 hospitalized patients in the USA treated with steroids, showing no significant difference in mortality with remdesivir, but a lower risk of acute kidney injury. | ||
Aug 5 2021 |
et al., Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD014962 | Remdesivir for the treatment of COVID-19 |
| Review of 5 RCTs prior to April 17, 2021 showing mortality RR 0.93 [0.81-1.06] for hospitalized patients. | ||
Jul 19 2021 |
et al., medRxiv, doi:10.1101/2021.07.15.21260600 | Remdesivir for the treatment of COVID-19 disease: A retrospective comparative study of patients treated with and without Remdesivir |
| 44% lower mortality (p=0.03). Retrospective 1,262 hospitalized patients, 398 treated with remdesivir, showing unadjusted lower mortality with treatment, and a treatment delay-response relationship. | ||
Jul 15 2021 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.14741 | Association of Remdesivir Treatment With Survival and Length of Hospital Stay Among US Veterans Hospitalized With COVID-19 |
| 6% higher mortality (p=0.66) and 100% longer hospitalization (p=0.001). Retrospective 5,898 hospitalized patients in the USA, 2,374 receiving remdesivir treatment, showing no significant difference in mortality, and a longer time to hospital discharge with treatment. | ||
Jul 13 2021 |
et al., Annals of Internal Medicine, doi:10.7326/M21-0653 | Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19 |
| no change in mortality (p=1). Small RCT in Norway with 52 HCQ and 42 remdesivir patients, showing no significant differences with treatment. Add-on trial to WHO Solidarity. NCT04321616. | ||
Jun 21 2021 |
et al., medRxiv, doi:10.1101/2021.06.18.21259072 | Evaluation of the effectiveness of remdesivir in treating severe COVID-19 using data from the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, national cohort study |
| 20% lower mortality (p=0.03) and 68% higher ventilation (p=0.003). Prospective PSM analysis of remdesivir use in the UK showing statistically significantly lower mortality at 28 days. For unspecified reasons, the study prioritized short-term outcomes. Mortality at 14 days was also lower but not statistic.. | ||
May 26 2021 |
et al., Research Square, doi:10.21203/rs.3.rs-365321/v2 | Case Characteristics, Clinical Data, And Outcomes of Hospitalized COVID-19 Patients In Qom Province, Iran: A Prospective Cohort Study |
| 2% higher mortality (p=0.92). Prospective study of 2,468 hospitalized COVID-19 patients in Iran, showing no significant difference with remdesivir treatment. IR.MUQ.REC.1399.013. | ||
May 4 2021 |
et al., Journal of the Formosan Medical Association, doi:10.1016/j.jfma.2021.04.026 | Assessing Efficacy of Antiviral Therapy for COVID-19 Patients: A Case Study on Remdesivir with Bayesian Synthesis Design and Multistate Analysis |
| Bayesian synthesis design and multistate analysis of remdesivir results showing 31% [18-44%] lower risk of death and 10% [1-18%] higher recovery. | ||
Apr 30 2021 |
et al., PLOS ONE, doi:10.1371/journal.pone.0251085 | Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: A prospective multi-center cohort study |
| 11% higher mortality (p=0.65). Prospective study of 465 COVID-19 ICU patients in Libya showing no significant differences with treatment. | ||
Apr 29 2021 |
et al., Journal of Medical Virology, doi:10.1002/jmv.27053 | Decreased In-Hospital Mortality Associated with Aspirin Administration in Hospitalized Patients Due to Severe COVID-19 |
| 19% lower mortality (p=0.49). Retrospective 991 hospitalized patients in Iran focusing on aspirin use but also showing results for HCQ, remdesivir, and favipiravir. | ||
Apr 7 2021 |
et al., BMJ Open, doi:10.1136/bmjopen-2020-042042 | 3219 hospitalised patients with COVID-19 in Southeast Michigan: a retrospective case cohort study |
| 86% higher mortality (p=0.54). Retrospective database analysis of 3,219 hospitalized patients in the USA. Very different results in the time period analysis (Table S2), and results significantly different to other studies for the same medications (e.g., heparin OR 3.06.. | ||
Mar 20 2021 |
et al., Indian Journal of Anasthesia, doi:10.4103/ija.IJA_149_21 | Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: A prospective randomised study |
| 76% higher mortality (p=0.47) and 112% higher ventilation (p=0.42). Small RCT with 34 remdesivir patients and 36 controls finding no significant difference in clinical outcomes. | ||
Mar 10 2021 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.02.039 (date from preprint) | Efficacy of remdesivir in hospitalized nonsevere COVID-19 patients in Japan: A large observational study using the COVID-19 Registry Japan |
| 4% higher mortality (p=0.21), 2% lower ventilation (p=0.68), and 15% lower progression (p=0.68). Retrospective database analysis of 12,487 hospitalized patients in Japan, showing lower risk of oxygen requirement, but no significant difference in mortality or ventilation/ECMO. | ||
Mar 9 2021 |
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2021.02.029 | A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary center in Israel |
| 9% shorter hospitalization (p=0.77) and no change in viral clearance (p=0.98). Retrospective 29 remdesivir patients and 113 controls, not finding a significant difference in nasopharyngeal viral load or hospitalization time. Hospitalization time was lower with treatment, with a larger reduction for non-intubated pat.. | ||
Mar 8 2021 |
et al., medRxiv, doi:10.1101/2021.03.05.21251351 | A single-center retrospective cohort study of Covid-19 medications: Remdesivir, Favipiravir, Methylprednisolone, Dexamethasone, and Interferon β1a and their combinations |
| Retrospective 324 hospitalized patients in Iran reporting on the use remdesivir, favipiravir, methylprednisolone, dexamethasone, and interferon β1a and their combinations. There is no control group in this study, however authors suggest t.. | ||
Feb 28 2021 |
et al., Gastroenterology, doi:10.1053/j.gastro.2020.10.011 | Famotidine Use Is Not Associated With 30-day Mortality: A Coarsened Exact Match Study in 7158 Hospitalized Patients With Coronavirus Disease 2019 From a Large Healthcare System |
| 24% higher mortality (p=0.87). Retrospective 7,158 hospitalized COVID-19 patients in the USA analyzing famotidine treatment, showing no significant difference in mortality with associated remdesivir treatment. | ||
Feb 27 2021 |
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2021.02.013 | Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns |
| Comparison of bradycardia in COVID-19 patients treated with remdesivir compared to those treated with HCQ, lopinavir/ritonavir, tocilizumab or glucocorticoids, finding increased risk of bradycardia with remdesivir. | ||
Dec 28 2020 |
et al., Fundamentals of Clinical Pharmacology, doi:10.1111/fcp.12643 | Remdesivir potently inhibits carboxylesterase-2 through covalent modifications: signifying strong drug-drug interactions |
| Analysis finding that remdesivir at nanomolar concentrations inhibits carboxylesterase-2 (CES2) through covalent modifications. CES2 is a major drug metabolizing enzyme. Authors conclude that caution must be exercised when remdesivir is u.. | ||
Dec 24 2020 |
et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542 (date from preprint) | Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2 |
| In Vitro study showing enhanced antiviral activity of ivermectin and remdesivir in combination. | ||
Dec 19 2020 |
et al., Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145 | Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database |
| VigiBase retrospective showing a 20x increase in the risk of acute renal failure with remdesivir. | ||
Nov 29 2020 |
et al., International Journal of Sciences, doi:10.18483/ijSci.2417 | Efficacy of Remdesivir in Covid-19 Patients; Multicenter Study in Lahore |
| 100% higher mortality (p=0.33) and 250% higher ventilation (p=0.15). Small late stage (hospitalized, <12 days symptoms) remdesivir study showing non-statistically significant higher mortality with treatment. No adjustments were made for differences in the groups. Remdesivir mean age was 49 vs. control 57. .. | ||
Nov 20 2020 |
et al., medRxiv, doi:10.1101/2020.11.19.20234153 | Effectiveness of remdesivir with and without dexamethasone in hospitalized patients with COVID-19 |
| 20% lower mortality (p=0.44) and 35% greater improvement (p<0.0001). Retrospective 303 remdesivir patients and 303 matched controls showing significantly faster clinical improvement, and lower (but not statistically significant) mortality. | ||
Nov 3 2020 |
et al., Polish Archives of Internal Medicine, doi:10.20452/pamw.15735 | Remdesivir-based therapy improved recovery of patients with COVID-19 in the SARSTer multicentre, real-world study |
| 49% lower mortality (p=0.18) and 56% greater improvement (p=0.01). Retrospective study comparing 122 remdesivir patients and 211 lopinavir/ritonavir patients, showing higher rates of clinical improvement with remdesivir and lower mortality (not statistically significant). | ||
Oct 20 2020 |
et al., Journal of Intensive Care Medicine, doi:10.1177/0885066621994476 (date from preprint) | Clinical Course and Outcome of COVID-19 Acute Respiratory Distress Syndrome: Data From a National Repository |
| 29% lower mortality (p=0.03). Retrospective 7,816 Veterans Affairs hospitalized patients showing lower mortality with remdesivir. | ||
Oct 15 2020 |
, NEJM, doi:10.1056/NEJMoa2023184 (date from preprint) | Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results |
| 5% lower mortality (p=0.53). WHO SOLIDARITY open-label RCT with 2,750 very late stage (76% on oxygen/ventilation) remdesivir patients, mortality relative risk RR 0.95 [0.81-1.11], p=0.50. Non-ventilated patients show a greater benefit, RR 0.86 [0.72-1.04], p = 0.13. | ||
Oct 8 2020 |
et al., NEJM, doi:10.1056/NEJMoa2007764 | Remdesivir for the Treatment of Covid-19 — Final Report |
| 27% lower mortality (p=0.07) and 22% improved recovery (p=0.0005). RCT 1,062 hospitalized patients showing faster recovery time with treatment, median 10 days vs. 15 days for placebo, rate ratio for recovery 1.29, p<0.001. Day 29 mortality was 11.4% with remdesivir and 15.2% with placebo, hazard ratio HR.. | ||
Aug 28 2020 |
et al., Clinical Infectious Disease, doi:10.1093/cid/ciaa1268 | Patient Characteristics and Outcomes of 11,721 Patients with COVID19 Hospitalized Across the United States |
| 61% lower mortality (p=0.02) and 37% higher ventilation (p=0.25). Database analysis of 11,721 hospitalized patients, 48 treated with remdesivir. Data inconsistencies have been found in this study, for example 99.4% of patients treated with HCQ were treated in urban hospitals, compared to 65% of untreate.. | ||
Aug 23 2020 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkaa321 | Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU |
| 16% lower mortality (p=0.03). Retrospective 51 ICU patients under mechanical ventilation, 25 treated with remdesivir, showing lower mortality with treatment. | ||
Aug 21 2020 |
et al., JAMA, doi:10.1001/jama.2020.16349 | Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19A Randomized Clinical Trial |
| 35% lower mortality (p=0.5). Late stage (median 8 days from symptom onset) RCT 584 patients with moderate COVID-19 showing (non-statistically significant) lower mortality. 5-day remdesivir had significantly higher odds of a better clinical status distribution on the .. | ||
Jul 24 2020 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaa1041 | Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care |
| 59% lower mortality (p=0.001). Comparative analysis between remdesivir trial GS-US-540–5773 and a retrospective SOC cohort with similar inclusion criteria, showing lower mortality and higher recovery at day 14 with remdesivir. | ||
Jun 30 2020 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.06.093 | Case report study of the first five COVID-19 patients treated with remdesivir in France |
| Early report on 5 ICU patients treated with remdesivir highlighting issues with usage in critically ill patients. | ||
Jun 29 2020 |
et al., medRxiv, doi:10.1101/2020.06.22.20136531 | Efficacy and Safety of Remdesivir for COVID-19 Treatment: An Analysis of Randomized, Double-Blind, Placebo-Controlled Trials |
| 36% lower mortality (p=0.02). Meta analysis of Beigel and Wang RCTs showing remdesivir significantly decreased mortality (8.18% vs. 12.70%, RR 0.64 [0.44-0.92], p = 0.175). | ||
May 27 2020 |
et al., NEJM, doi:10.1056/NEJMoa2015301 | Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 |
| RCT of remdesivir for 5 or 10 days with no placebo control group, showing no significant differences between 5 and 10 day treatment. NCT04292899. | ||
Apr 29 2020 |
et al., Lancet, doi:10.1016/S0140-6736(20)31022-9 | Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial |
| 9% higher mortality (p=1). Small RCT with 237 hospitalized patients in China with severe COVID-19, not showing statistically significant benefits. 158 treatment patients and 79 control patients. While too small for significance, the subgroup treated within 10 days .. | ||
Apr 10 2020 |
et al., NEJM, doi:10.1056/NEJMoa2007016 | Compassionate Use of Remdesivir for Patients with Severe Covid-19 |
| Report on compassionate use of remdesivir with 61 severe COVID-19 patients, showing clinical improvement in 36 of 53 patients. | ||
Please send us corrections, updates, or comments.
c19early involves the extraction of 100,000+ datapoints from
thousands of papers. Community updates
help ensure high accuracy.
Treatments and other interventions are complementary.
All practical, effective, and safe
means should be used based on risk/benefit analysis.
No treatment or intervention is 100% available and effective for all current
and future variants.
We do not provide medical advice. Before taking any medication,
consult a qualified physician who can provide personalized advice and details
of risks and benefits based on your medical history and situation. FLCCC and WCH
provide treatment protocols.
Thanks for your feedback! Please search before submitting papers and note
that studies are listed under the date they were first available, which may be
the date of an earlier preprint.